Most Abundant Chemical Sedimentary Rock
Chapter half dozen Sediments and Sedimentary Rocks
6.2 Chemical Sedimentary Rocks
Whereas clastic sedimentary rocks are dominated past components that have been transported equally solid clasts (clay, silt, sand, etc.), chemical sedimentary rocks are dominated by components that have been transported as ions in solution (Na+, Ca2+, HCO3 –, etc.). There is some overlap betwixt the two because almost all clastic sedimentary rocks contain cement formed from dissolved ions, and many chemic sedimentary rocks include some clasts. Since ions can stay in solution for tens of thousands of years (some much longer), and tin travel for tens of thousands of kilometres, information technology is almost impossible to relate chemical sediments back to their source rocks.
The most common chemic sedimentary rock, past far, is limestone. Others include chert, banded iron germination, and a diverseness of rocks that class when bodies of water evaporate. Biological processes are important in the formation of some chemical sedimentary rocks, peculiarly limestone and chert. For example, limestone is made upwards about entirely of fragments of marine[1] organisms that manufacture calcite for their shells and other difficult parts, and almost chert includes at least some of the silica tests (shells) of tiny marine organisms (such as diatoms and radiolaria).
Limestone
Virtually all limestone forms in the oceans, and near of that forms on the shallow continental shelves, specially in tropical regions with coral reefs. Reefs are highly productive ecosystems populated by a wide range of organisms, many of which utilize calcium and bicarbonate ions in seawater to make carbonate minerals (particularly calcite) for their shells and other structures. These include corals, of course, but also green and red algae, urchins, sponges, molluscs, and crustaceans. Especially subsequently they die, but fifty-fifty while they are still alive, these organisms are eroded by waves and currents to produce carbonate fragments that accumulate in the surrounding region, as illustrated in Figure vi.9.

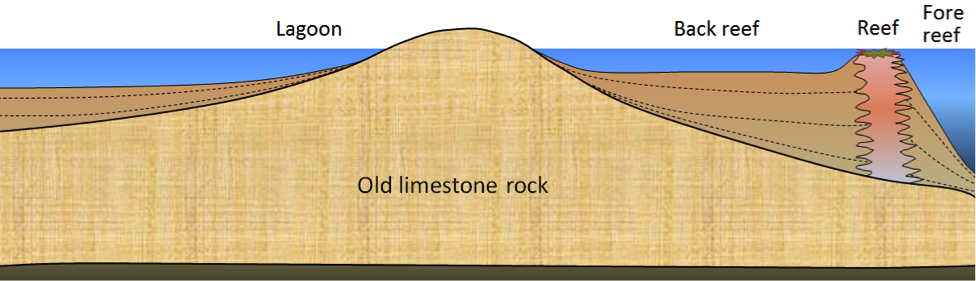
Figure 6.10 shows a cross-department through a typical reef in a tropical environs (normally between 40° N and 40° Southward). Reefs tend to form near the edges of steep drib-offs because the reef organisms thrive on nutrient-rich upwelling currents. Every bit the reef builds up, it is eroded by waves and currents to produce carbonate sediments that are transported into the steep offshore fore-reef area and the shallower inshore dorsum-reef area. These sediments are dominated by reef-blazon carbonate fragments of all sizes, including mud. In many such areas, carbonate-rich sediments as well accumulate in quiet lagoons, where mud and mollusc-trounce fragments predominate (Effigy half dozen.11a) or in offshore areas with strong currents, where either foraminifera tests accumulate (Figure half dozen.11b) or calcite crystallizes inorganically to grade ooids – spheres of calcite that grade in shallow tropical ocean water with strong currents (Figure 6.11c).

Limestone also accumulates in deeper h2o, from the steady rain of the carbonate shells of tiny organisms that lived near the body of water surface. The lower limit for limestone accumulation is around 4,000 m. Beneath that depth, calcite is soluble so limestone does not accrue.
Calcite tin also grade on land in a number of environments. Tufa forms at springs (Figure 6.12) and travertine (which is less porous) forms at hot springs. Like cloth precipitates inside limestone caves to form stalactites, stalagmites, and a wide range of other speleothems.

Dolomite (CaMg(COthree)2) is another carbonate mineral, but dolomite is as well the name for a stone composed of the mineral dolomite (although some geologists use the term dolostone to avoid defoliation). Dolomite rock is quite mutual (there's a whole Italian mountain range named after it), which is surprising since marine organisms don't make dolomite. All of the dolomite found in ancient rocks has been formed through magnesium replacing some of the calcium in the calcite in carbonate muds and sands. This procedure is known as dolomitization, and it is thought to accept place where magnesium-rich water percolates through the sediments in carbonate tidal flat environments.
Chert and Banded Atomic number 26 Formation
Every bit we've seen, not all marine organisms make their hard parts out of calcite; some, like radiolaria and diatoms, apply silica, and when they die their tiny shells (or tests) settle slowly to the bottom where they accrue as chert. In some cases, chert is deposited along with limestone in the moderately deep ocean, but the ii tend to remain separate, then chert beds within limestone are quite common (Figure 6.13), as are nodules, link the flint nodules of the Cretaceous chalk of southeastern England. In other situations, and especially in very deep water, chert accumulates on its own, commonly in thin beds.

Some ancient chert beds — most dating to betwixt 1800 and 2400 Ma — are also combined with a rock known as banded iron formation (BIF), a deep sea-floor deposit of iron oxide that is a common ore of iron (Effigy 6.fourteen). BIF forms when atomic number 26 dissolved in seawater is oxidized, becomes insoluble, and sinks to the bottom in the aforementioned way that silica tests practise to grade chert. The prevalence of BIF in rocks dating from 2400 to 1800 Ma is due to the changes in the temper and oceans that took place over that time menstruum. Photosynthetic bacteria (i.east., cyanobacteria, a.k.a. blue-light-green algae) consume carbon dioxide from the atmosphere and employ solar energy to convert information technology to oxygen. These bacteria first evolved around 3500 Ma, and for the adjacent billion years, almost all of that free oxygen was used up by chemic and biological processes, but by 2400 Ma free oxygen levels started to increase in the temper and the oceans. Over a period of 600 million years, that oxygen gradually converted soluble ferrous iron (Fetwo+) to insoluble ferric iron (Ironiii+), which combined with oxygen to form the mineral hematite (Fe2O3), leading to the accumulation of BIFs. After 1800 Ma, little dissolved atomic number 26 was left in the oceans and the germination of BIF essentially stopped.

Evaporites
In arid regions, lakes and inland seas typically have no stream outlet and the water that flows into them is removed only by evaporation. Under these atmospheric condition, the water becomes increasingly concentrated with dissolved salts, and eventually some of these salts reach saturation levels and start to crystallize (Figure 6.15). Although all evaporite deposits are unique because of differences in the chemical science of the water, in almost cases minor amounts of carbonates outset to precipitate when the solution is reduced to about 50% of its original volume. Gypsum (CaSOiv·HiiO) precipitates at nigh 20% of the original volume and halite (NaCl) precipitates at ten%. Other important evaporite minerals include sylvite (KCl) and borax (NatwoB4O7·10HiiO). Sylvite is mined at numerous locations across Saskatchewan (Figure 6.xvi) from evaporites that were deposited during the Devonian (~385 Ma) when an inland ocean occupied much of the region.


Practice 6.3 Making Evaporite
This is an easy experiment that y'all can do at home. Cascade nearly l mL (just less than 1/4 cup) of very hot water into a cup and add together 2 teaspoons (10 mL) of table salt. Stir until all or almost all of the salt has dissolved, then cascade the salty h2o (leaving any undissolved table salt behind) into a shallow wide dish or a small-scale plate. Leave it to evaporate for a few days and observe the outcome.
It may wait a footling like the photo here. These crystals are up to about 3 mm across.

Attributions
Effigy 6.11
JoultersCayOoids Past Wilson44691 nether Public domain.
Figure 6.fourteen
Dales Goge by Graeme Churchard nether CC BY 2.0.
Effigy six.16
Photo courtesy of PotashCorp, used with permission
Most Abundant Chemical Sedimentary Rock,
Source: https://opentextbc.ca/geology/chapter/6-2-chemical-sedimentary-rocks/
Posted by: smithcoctur.blogspot.com


0 Response to "Most Abundant Chemical Sedimentary Rock"
Post a Comment