Molar Mass Of Sugar C6h12o6
Glucose (C6H12O6) is an organic macromolecule that is essential for the metabolism of essentially all eukaryotic organisms. Glucose is a monosaccharide (uncomplicated sugar) and is the most abundant saccharide. Glucose is normally stored in the trunk in the course of starch or glycogen. Glucose provides the raw materials needed for cellular respiration and the production of ATP.
"The brain's preferred source of fuel is glucose/carbohydrates. And when yous go on a low-carb/loftier-poly peptide diet, your brain is using depression-octane fuel. Yous'll exist a piffling groggy, a little grumpy." — Jack LaLanne
The tooth mass of glucose can be calculated past multiplying the molar masses of its atomic constituents by their frequency in a single molecule and adding those values together. Glucose is composed of hydrogen (H), carbon (C), and oxygen (O) The molar mass of H is 1.0079, the molar mass of C is 12.0107, and the molar mass of O is 15.9994. In one molecule of glucose, in that location are 12 hydrogen, 6 carbon, and vi oxygen atoms. And then, altogether, the molar mass of a single molecule of glucose is equal to:
one.0079(12)+12.0107(vi)+15.9994(six) = 180.16 m/mol
Glucose has a tooth mass of 180.16 g/mol. One mole of glucose molecule has a mass of 180.16 grand.
Molar Mass
The molar mass of a given substance is a quantitative measure that tells you the mass of 1 mole of that substance. In chemistry, tooth mass is understood as a physical holding that is defined as the mass of a substance divided by the amount of that substance.
The measure out of molar mass (g/mol) is based on the SI unit for quantity, themole(not to be confused with the cute burrowing mammal). 1 mole is defined every bit an amount of substance that contains exactly 6.0221476 × 1023 elective particles. Merely similar the words "1000000" and "billion," the word "mole" stands for a specific quantity of things; approximately 602,214,150,000,000,000,000,000 of them. If I had 1 mole of apples, I would have 602,214,150,000,000,000,000,000 apples, if I had one mole of hydrogen atoms, I would have 602,214,150,000,000,000,000,000 of them.
Every element has a molar mass, i.e. a measure of how much mass one mole of that element has. The molar mass of whatsoever element can be determined by multiplying that elements standard atomic weight (listed on the periodic table) past the molar mass constant Mu=1g/mol. Hydrogen, for case, has a standard atomic weight of one.00794. To notice the molar mass of hydrogen, we simply multiply this number by the molar mass constant to go 1.00794 g/mol. So, hydrogen has a molar mass of 1.00794 g/mol; that is, six.0221476 × 1023hydrogen atoms would together counterbalance i.00794 grams.
To observe the tooth mass of a molecule or an ionic compound, all one has to exercise is first multiply the molar masses of the constituent elements by their frequency in the compound, and add the total values together. I tin can make up one's mind the relative diminutive frequencies of a composition by the compound's molecular formula. In other words, the tooth mass of a compound is equal to the sum of the molar masses of its constituent atoms.
"The production and consumption of glucose, and hence, the blood sugar level, are controlled by a functional endocrine equilibrium." — Bernardo Houssay
For instance, water is made of 2 hydrogen atoms and ane oxygen cantlet and has a molecular formula of H2O. To find the molar mass of water, one starting time needs to find the tooth mass of hydrogen and oxygen, multiply those values by their relative frequency in a unmarried molecule of the compound, and add together the totals together. Hydrogen has a molar mass of 1.00794 and oxygen has a molar mass of 15.9994. Each molecule of water has 2 hydrogen atoms and 1 oxygen atom, so the molar mass of water equals:
1.00794(2) + 15.9994(1) ≈ eighteen.02 thousand/mol
And then one mole of water molecules would have a weight of 18.02 grams.
Importance Of Tooth Mass
Tooth masses are important because they figure in equations used to predict the physical and chemic behavior of substances. Nearly importantly, the concept of molar mass serves as the bridge betwixt mass and corporeality of substance because information technology is generally impossible to directly count how many particles are in a substance. Wecan measure out mass though, so knowing the molar mass allows united states to indirectly measure the number of particles in a substance by measuring its mass.
Experimental setups often reference moles and molar masses in their steps. Say an experiment calls for 3 moles of water. Nosotros cannot directly count individual molecules of water (it would take style too long even if we could) then instead nosotros can rely on the molar mass of water to figure out how much water we need. i mole of water has a mass of 18.02 grams, and so if an experiment calls for 3 moles of water, nosotros know that nosotros need 18.02(three) = 54.06 grams of water. Likewise, if an experiment called for 0.7 moles of carbon, we know that nosotros demand 12.0107(0.7) = 8.407 grams of carbon.
Tooth Mass Vs Molecular Mass
It is important to non misfile the concepts of molar mass and molecular mass. The molar mass of a compound tells you how much one mole of a substance weighs but it does not really tell you annihilation nigh the weights of the individual molecules. The measure of the mass of an individual molecule of a compound is itsmolecular mass. Molecular masses are measured indaltons (Da), named after the father of atomic theory, John Dalton. Molecules of the same chemical compound tin have different molecular masses because they can be composed of different isotopes of the same chemical element. Water may have a tooth mass of 18.02 thousand/mol, but individual water molecules can have a weight that ranges from 18.011 Da to 22.028 Da, due to the presence of different isotopes of hydrogen and oxygen. The molar mass tin so exist seen equally a measure of the boilerplate molecular masses of the private molecules in one mole of a substance.
Molar Mass Of Glucose
Using the above definitions, we can make up one's mind the molar mass of glucose step by step. First, we look at the molecular formula to determine the diminutive constituents and their relative frequencies in a unmarried molecule. glucose has a molecular formula of Chalf-dozenH12O6, so a single molecule of glucose contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
The molar masses of carbon, hydrogen, and oxygen are 12.0107 yard/mol, one.00794 g/mol, and 15.9994 g/mol, respectively. These values can exist determined by multiplying the standard atomic weight for each element by the molar mass abiding. Side by side, we tin can multiply these values by the frequency of each element, so:
12.0107 × vi
one.00794 × 12
15.9994 × six
Calculation all these values together will requite us the total molar mass of glucose:
1.0079(12)+12.0107(half-dozen)+15.9994(six) = 180.16 g/mol
Glucose As A Chemical compound
Glucose is a simple carbohydrate (monosaccharide) that is ubiquitous in living organisms. It is the master source of metabolic energy in well-nigh all living creatures and is physically abundant in many structures in the torso. Glucose is classified as a hexose (6 carbon atoms) and has several singled-out polymorphs. The most common and naturally occurring course, D-glucose, consists of a cyclical chain of v carbon atoms each bonded to a hydrogen and hydroxyl group, closed off with a carbon-containing aldehyde group (R[CHO]). In certain solutions, glucose will unravel from its cyclical organisation to form a linear chain of carbon atoms capped with the aldehyde group.
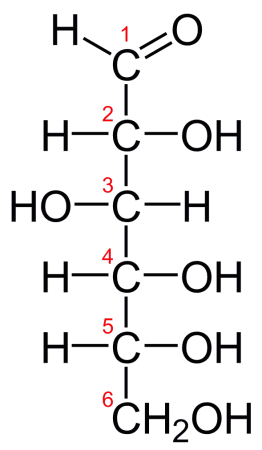
Fischer projection of D-glucose. Credit: "D-glucose chain" via WikiCommons CC0 i.0
All kinds of glucose are colorless and are easily dissolved in h2o, alcohol, and other organic solvents. Its solubility makes it an essential chemical compound for biological processes. Photoautotrophs, like plants, produce their own source of glucose via photosynthesis, but heterotrophs, like humans and all other mammals, must get their glucose from external sources. Glucose is the main ingredient that gets candy during cellular respiration.
During cellular respiration, i glucose molecule is cleaved downwardly into two pyruvate molecules in a process called glycolysis. The pyruvate molecules are then converted into acetyl-CoA, which is processed according to the Krebs cycle. The energy produced during the Krebs cycle is the master driver of oxidative phosphorylation, the procedure by which the body actually produces ATP, the fundamental energy currency of biochemical processes. ATP drives literally every biological reaction in the body, and then without a steady supply of glucose, the body will not exist able to produce its fuel. For every 1 molecule of glucose, one full plow of the cellular respiration cycle has a theoretical yield of 38 molecules of ATP. In practice, inefficiencies in chemical reactions or loss of energy during oxidative phosphorylation give an actual yield of nigh 33-34 molecules of ATP per molecule of glucose.
Glucose in the claret is called claret saccharide. Normal actual operation requires some level of blood carbohydrate, but too much can exist harmful. Elevated levels of claret carbohydrate, called hyperglycemia, can pb to nausea, fatigue, stomach pains, blurred vision, and frequent urination. Diabetics lack the ability to produce insulin, the hormone that regulated blood sugar levels, so diabetics are at risk for hyperglycemia. In severe cases, high levels of claret saccharide tin restrict oxygen flow through the capillaries, resulting in infection and tissue decease.
To recap, every element has a tooth mass, a measure of how much ane mole of that substance weighs. The tooth mass of an chemical element can be adamant by multiplying the standard atomic weight by the molar mass constant g/mol. The molar mass of a compound is equal to the sum of the molar masses of its elective elements. The tooth mass of a compound can exist determined by multiplying the molar masses of the individual elements by their relative frequency in a molecule of a compound and summing the full values. In the case of glucose (C6H12Ovi), glucose has a molar mass of180.xvi chiliad/mol.
Molar Mass Of Sugar C6h12o6,
Source: https://sciencetrends.com/molar-mass-of-glucose-c%E2%82%86h%E2%82%81%E2%82%82o%E2%82%86/
Posted by: smithcoctur.blogspot.com


0 Response to "Molar Mass Of Sugar C6h12o6"
Post a Comment